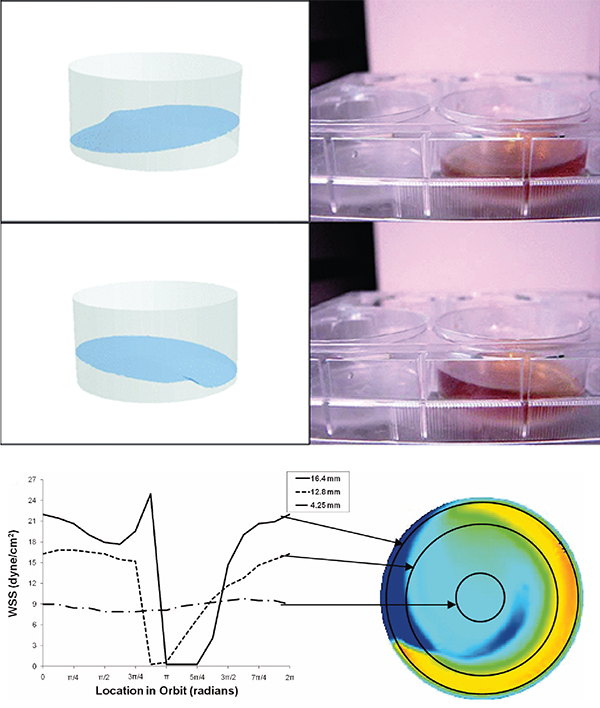
Figure 1. Orbital shaker for creation of shear stress. A – Movement of fluid inside a 6-well plate on the orbital shaker (adapted form Salek et al 2012). B – Wall shear stress (WSS) in the different regions of the well (adapted from Thomas et al. 2011).
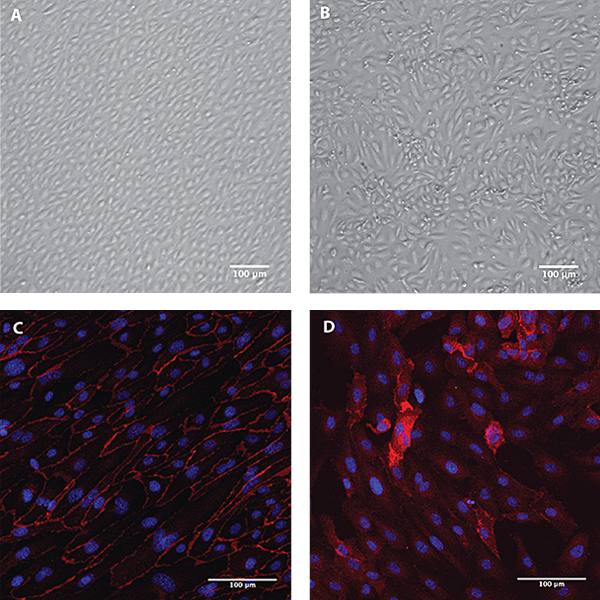
Figure 2. Endothelial cells under shear stress using an orbital shaker. Morphology and orientation of HUVECs after 8 days culturing on the orbital shaker (A) or under static conditions (B). Immunostaining for CD31 (red) and DAPI (blue) of human aortic endothelial cells after 8 days culturing on the orbital shaker (C) or under static conditions (D).
Andreia Fernandes
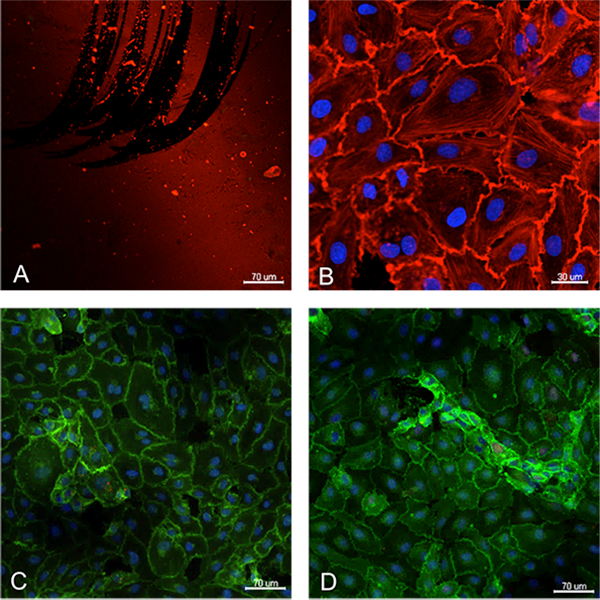
Figure 3. Assessment of endothelial cell growth and marker expression on top of the silicon membrane. The membrane was previously coated with fibronectin, shown in red (A). Endothelial cells grew with characteristic cobblestone morphology, as shown in B by the phalloidin staining (red) and expressed typical endothelial phenotype as confirmed by the expression of VE-cadherin (C) and CD31 (D). Cell nuclei are stained blue (DAPI).
Emanuela Fioretta
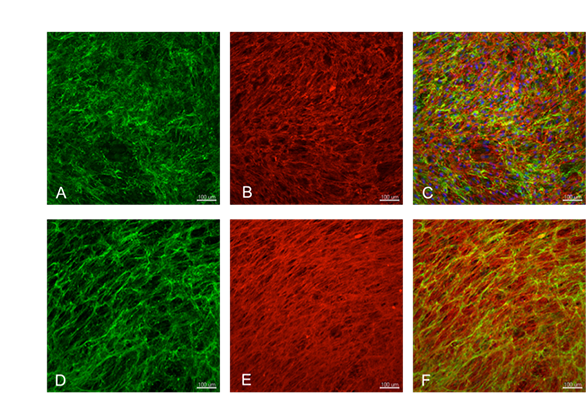
Figure 4. To enhance cell adhesion and growth on the membrane, novel coatings are investigated. Here, human derived myofibroblast cells have been cultured to induce extracelluar matrix deposition. The images A-C show that the cells have produced a homogeneous and abundant layer of collagen III (green) and fibronectin (red). Cell nuclei are stained blue by DAPI in the merged picture. To obtain the coating, removal of the tissue-producing cells is required. This process, called decellularization, was performed and the result is showed in images D-F. The layer of proteins was retained on the substrate while the cell component was removed (cell nuclei are not visible anymore in the merged picture). The substrate covered with the layer of extracellular matrix can then be used to enhance endothelial cell adhesion and growth.
Emanuela Fioretta
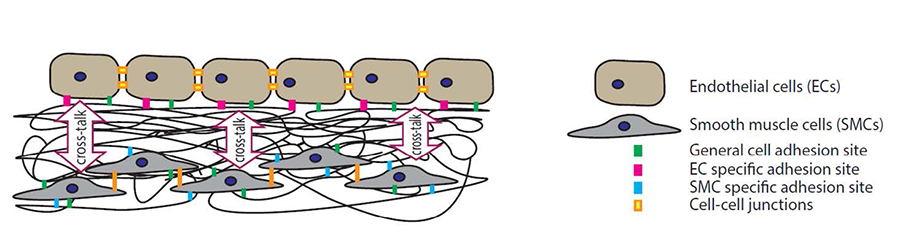
Figure 5. The aim is to identify cell-specific proteins/protein-sequences that mediate the adhesion of endothelial cells and smooth muscle cells to the substrate. Subsequently, we will create e-spun membranes presenting optimal densities and ratios of these proteins/protein-sequences to promote the formation of a stable and functional endothelial cell monolayer.